Allergan Asks To Dismiss Breast Implant Claims As Preempted
Allergan Asks To Dismiss Breast Implant Claims As Preempted
Introduction
Allergan USA Inc. filed a supplemental brief on January 5 in the U.S. District Court for the District of New Jersey, asking the federal judge to dismiss claims against its breast implants on the grounds that the consolidated lawsuits are preempted by federal law.
According to the memorandum that supports the motion, the company stated that complaints filed by the plaintiffs challenge the U.S. Food and Drug Administration’s (FDA) regulatory oversight that governs the design, manufacture, labeling, and post-sale reporting for the breast implants and breast tissue extenders.
With this memorandum, the company first took a shot on failure-to-warn claims, arguing that the premarket approval (PMA) only allows the manufacturer to make specific label changes and doesn't restrict them to do so. Additionally, the company added that the PMA only defines when it has to apply for a label change with the FDA, without requiring it to provide a warning to physicians or consumers.
The company argued that the plaintiff's aim on the FDA-approved process must be preempted as no allegations are stating that Allergan's manufacturing methods deviated from the approved PMA.
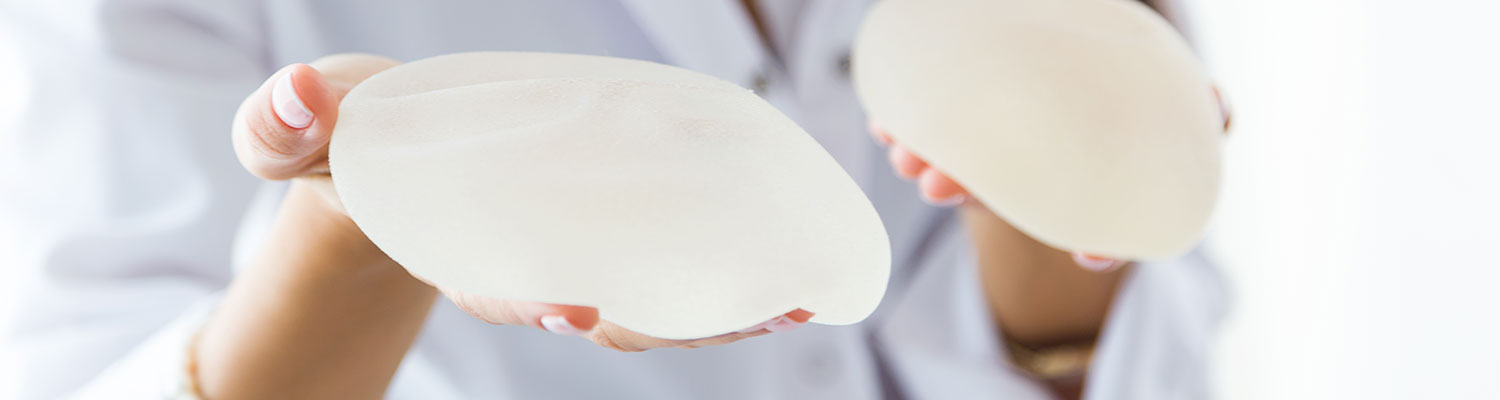
Breast implants are used in both breast augmentation surgery (to increase the breast size) and in breast reconstruction (to replace breast tissue that has been removed due to cancer or trauma or that has failed to develop properly due to a severe breast abnormality).
Allergan Inc.’s breast implants include saline and silicone devices used in breast augmentation and reconstruction. The company is one of the largest breast implant manufacturers in the world and started selling breast implants in 2006. Allergan’s flagship breast implant brand is Natrelle, but it also sells implants under its subsidiaries Inamed and McGhan. The manufacturer received approval from the FDA on 11/17/2006.
Currently, Allergan is facing nearly 150 product liability lawsuits and class action lawsuits over its breast implants, each claiming that the textured design was unreasonably dangerous and defective, and the manufacturer knew that it increased the risk of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) yet failed to warn about those risks.
The lawsuits are consolidated before U.S. District Judge Brian R. Martinotti in the District of New Jersey under MDL No.: 2921. Also, lawsuits filed in New Jersey are consolidated as part of multicounty litigation (MCL) in the New Jersey Superior Court for Bergen County, where the claims will be presided by Judge Rachelle Harz for coordinated discovery and pretrial proceedings.
Latest News
Texas Trial to Decide J&J’s $10B Talcum Powder Settlement
A high-stakes trial in Texas will determine whether Johnson & Johnson (J&J) can resolve tens of thousands of talcum powder cancer lawsuits through a…




