CDC Releases New Guidelines Over Opioid Prescriptions
CDC Releases New Guidelines Over Opioid Prescriptions
Introduction
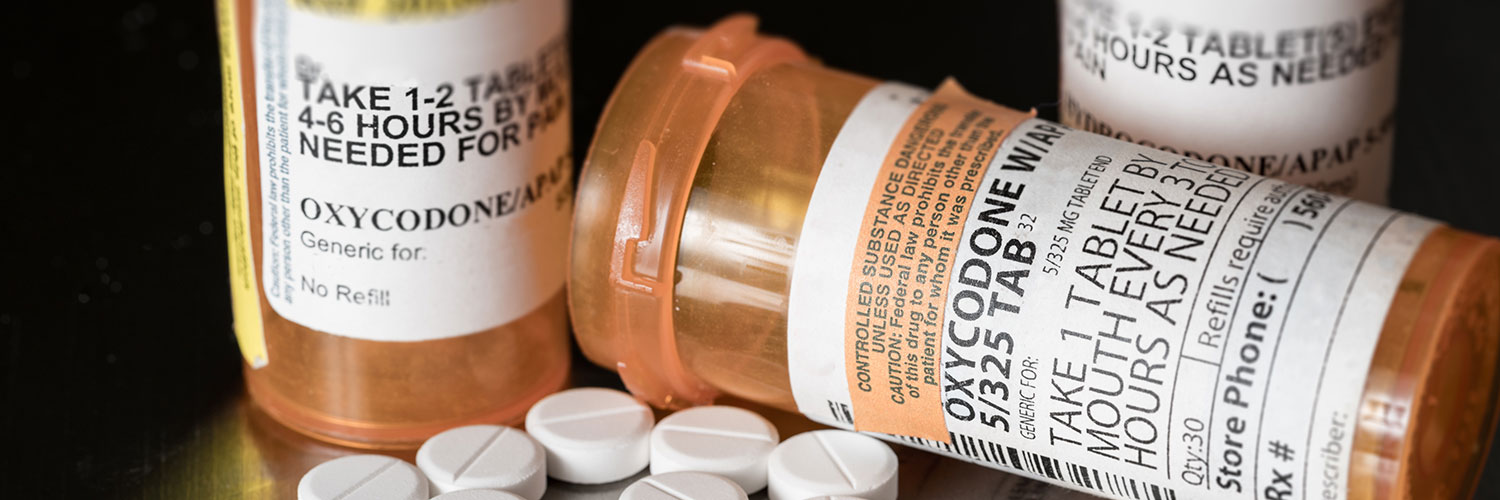
The Centers for Disease Control and Prevention has released new guidelines for clinicians on when and how to prescribe opioids for pain.
The new guidance replaces the agency's 2016 recommendations, which were criticized by some doctors and patients for encouraging an anti-opioid culture. According to the updated guidance, doctors, insurers, pharmacies, and regulators occasionally misapplied the older guidelines, resulting in significant harm to some patients, including untreated and undertreated pain, severe withdrawal symptoms, worsening pain outcomes, psychological distress, overdose, and suicide.
The 100-page document and its topline recommendation serve as a road map for prescribers navigating the difficult issue of treating pain, including advice on dealing with pain relief after surgery and managing chronic pain conditions, which are estimated to affect one in every five Americans.
The 2016 guidelines had a significant impact on policy, fueling a push by insurers, state medical boards, politicians, and federal law enforcement to reduce opioid prescribing.
Doctors and researchers say the fallout is difficult to overstate because it is a crisis of untreated pain. Many patients with severe chronic pain had their long-term prescriptions quickly reduced or discontinued, sometimes with disastrous consequences such as suicide or overdose, as they turned to a tainted supply of illicit drugs.
Federal agencies attempted to correct the course by emphasising that the older voluntary guidelines were not intended to become strict policies or laws. However, doctors and patient advocates hoped that the CDC's updated guidelines would reverse some of the unintended consequences of the previous guidance.
When the CDC announced the new clinical guidelines, it was clear that this was on their minds. The updated guidelines' co-author, the acting director of the CDC's National Center for Injury Prevention and Control, stated that the recommendations are voluntary and intended to guide shared decision-making between a clinician and a patient. It is not intended to be implemented as absolute policy or practise limits by clinicians, health systems, insurance companies, or government entities.
The new guidelines continue to emphasize that opioids should not be the first line of treatment in many cases, citing evidence that other treatments and approaches are frequently comparable for improving pain and function. The recommendations, however, make it clear that the guidance should not be used to replace clinical judgement and that clinicians can work with patients who are in pain, even if that means keeping them on opioids.
The new guidance's most significant changes come in the form of 12 bullet points that outline general prescribing principles.
Unlike the 2016 version, those takeaways no longer include specific limits on the dose and duration of an opioid prescription that a patient can take, though the document does warn against prescribing above a certain threshold further down. The new guidelines also expressly caution doctors against abruptly tapering or discontinuing opioid prescriptions for patients who are already on them, unless there are signs of a life-threatening problem.
Prescription restrictions became a policy or law as a result of previous guidelines. Even though the new guidelines state that they are not intended to be implemented as absolute limits for policy or practice, it is unclear whether those rules will be rewritten in light of the new guidelines.
Latest News
Texas Trial to Decide J&J’s $10B Talcum Powder Settlement
A high-stakes trial in Texas will determine whether Johnson & Johnson (J&J) can resolve tens of thousands of talcum powder cancer lawsuits through a…




