Sientra Faces Lymphoma Lawsuit Over Textured Breast Implants
Sientra Faces Lymphoma Lawsuit Over Textured Breast Implants
Introduction
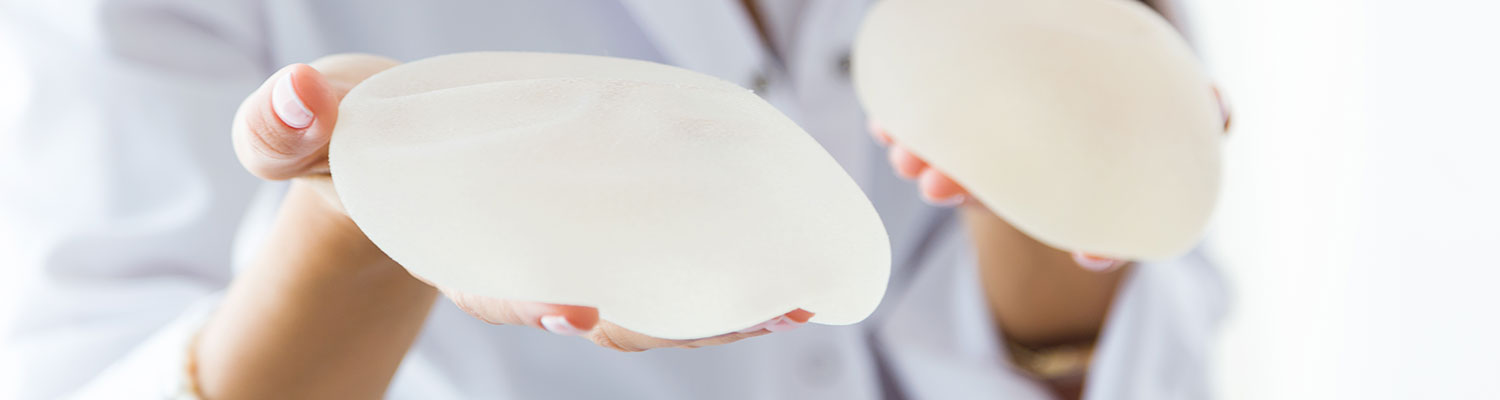
Sientra is facing allegations similar to Allergan over its silicone implants by a Tennessee woman who claims that the textured breast implants made in Brazil resulted in her breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), a rare type of cancer in the surrounding tissue.
The lawsuit was filed by Kelly Painter-Hart and her husband, Seth Hart, in the U.S. District Court for the Eastern District of Tennessee. According to the lawsuit filed, Painter-Hart had bilateral mastectomies in November 2013 as a precautionary measure over breast cancer and was implanted with Sientra textured oval-shaped silicone gel implants.
In September 2019, she went to her surgeon complaining of swelling in her right breast, and in October, she was diagnosed with BIA-ALCL, following which she removed the implants surgically a month later. The lawsuit claims that the couple and her surgeon were never warned of the risk associated with the textured breast implants.
The lawsuit further states that the implants were made by Silimed, and the manufacturer had acknowledged its failure to follow good manufacturing practices in a first-quarter report published in 2015. These practices result in negligently manufactured, overly textured, rough implant shells containing foreign and adulterated silicone particles, fragments, implant materials, and residues, which triggers T-cell lymphoma and over time, BIA-ALCL, the lawsuit claims.
The couple is seeking damages based on negligence, manufacturing defects, failure to warn, and breach of express and implied warranties for the pain and suffering, and the permanent physical impairment and disfigurement caused, along with punitive damages.
Allergan is also facing similar lawsuits over its recalled “Biocell” textured breast implants. The Food and Drug Administration (FDA) recently indicated that out of the 733 BIA-ALCL cases reported, where the manufacturer has been identified, 620 are linked to breast implants sold by Allergan.
I developed a rare cancer related to the Sientra textured implants and now I want to file a lawsuit.
Please contact me at 7867276122
Or by email [email protected]





Ive got textured breast implants in 2021 ive had pain in my right even up in the arm pit?