Weekly Mass Torts Bulletin 2024-April-29
MDL Judge Dismisses 4 Paraquat Cases, Excludes Expert

The U.S. District Judge overseeing the Paraquat Parkinson’s disease lawsuits has barred a key expert witness from testifying at trial, leading to the dismissal of four bellwether cases lacking the necessary support to proceed.
These cases are part of a larger litigation against Syngenta and Chevron, encompassing over 5,000 lawsuits alleging Paraquat exposure causes Parkinson’s disease. Plaintiffs contend manufacturers failed to sufficiently warn about Paraquat risks during handling.
Expert witnesses were proposed to validate the Paraquat-Parkinson’s link, citing research indicating Paraquat's potential to induce the disease. However, manufacturers refute these assertions, maintaining the herbicide's safety.
All federal Paraquat lawsuits are centralized in the Southern District of Illinois as multidistrict litigation. Bellwether trials, intended to predict jury responses, are crucial components of this process.
During the Paraquat “Daubert” hearings, the Judge evaluated the proposed expert testimony's reliability. Defendants sought the exclusion of an epidemiologist and statistician from Cornell University, pivotal in establishing causation in four bellwether cases.
In a subsequent memorandum and order, the Judge sided with the defendants, excluding the expert testimony due to insufficient evidence linking Paraquat to Parkinson’s disease. As this expert was key to plaintiffs’ causation arguments, all four bellwether trials were dismissed.
This ruling underscores the litigation’s complexity and the challenges associated with proving causation in Paraquat-related Parkinson’s disease cases. While these developments represent setbacks for plaintiffs, the legal process continues, with further proceedings expected in the multidistrict litigation.
Study: Vaping Equals Smoking in DNA Damage

A recent study conducted by Ecuadorian researchers has added to the growing body of evidence suggesting that vaping electronic cigarettes may pose similar risks to smoking traditional tobacco cigarettes.
The study found that individuals who vape, regardless of whether their e-cigarettes contain tobacco or nicotine, face a comparable level of DNA damage to those who smoke tobacco cigarettes.
Traditionally, tobacco cigarettes are known to contain around 70 different types of chemicals, many of which are carcinogenic and can lead to DNA damage. E-cigarettes, on the other hand, may contain even more cancer-causing chemicals, with some estimates suggesting over 80 such compounds. Despite the perception that vaping is a "healthier alternative" to smoking, numerous studies have indicated that e-cigarettes deliver significant amounts of tobacco and other toxic substances, potentially leading to severe health consequences, including cancer.
In this latest study, researchers evaluated chromosomal damage in various groups of individuals, including traditional cigarette smokers, vapers using nicotine-containing e-cigarettes, vapers using nicotine-free e-cigarettes, and a control group of non-smokers and non-vapers. The study involved 120 participants, and the results showed similar levels of DNA damage among all groups exposed to smoking or vaping. Individuals in all three smoking or vaping groups exhibited higher rates of chromosomal breaks and gaps compared to those who did not smoke or vape.
Prior research has consistently shown that smoking tobacco cigarettes can damage human DNA, increasing the risk of cancer later in life. Similarly, recent studies have suggested that vaping may also lead to changes in human DNA, posing comparable cancer risks. Additionally, vaping has been linked to an increased risk of cardiovascular disease and addiction, with growing concerns about its impact on teen health, including lung damage, cardiovascular issues, and mental health disorders like major depression.
In light of the mounting evidence suggesting that vaping may be as harmful as smoking tobacco cigarettes, consumer advocates are calling for enhanced public health measures to educate both smokers and vapers about the risks associated with these behaviors. The researchers emphasized the need for further studies and larger sample sizes to fully understand the genotoxic effects of e-cigarettes. They concluded that while e-cigarettes without nicotine may cause less DNA damage compared to those with nicotine, both types are still genotoxic. They underscored the importance of conducting educational and public health campaigns to raise awareness about the risks of vaping.
In summary, the study highlights the concerning similarity in DNA damage between smoking traditional tobacco cigarettes and vaping electronic cigarettes. It underscores the need for comprehensive public health interventions to inform individuals about the potential risks associated with both smoking and vaping, as they both pose significant threats to DNA integrity and overall health.
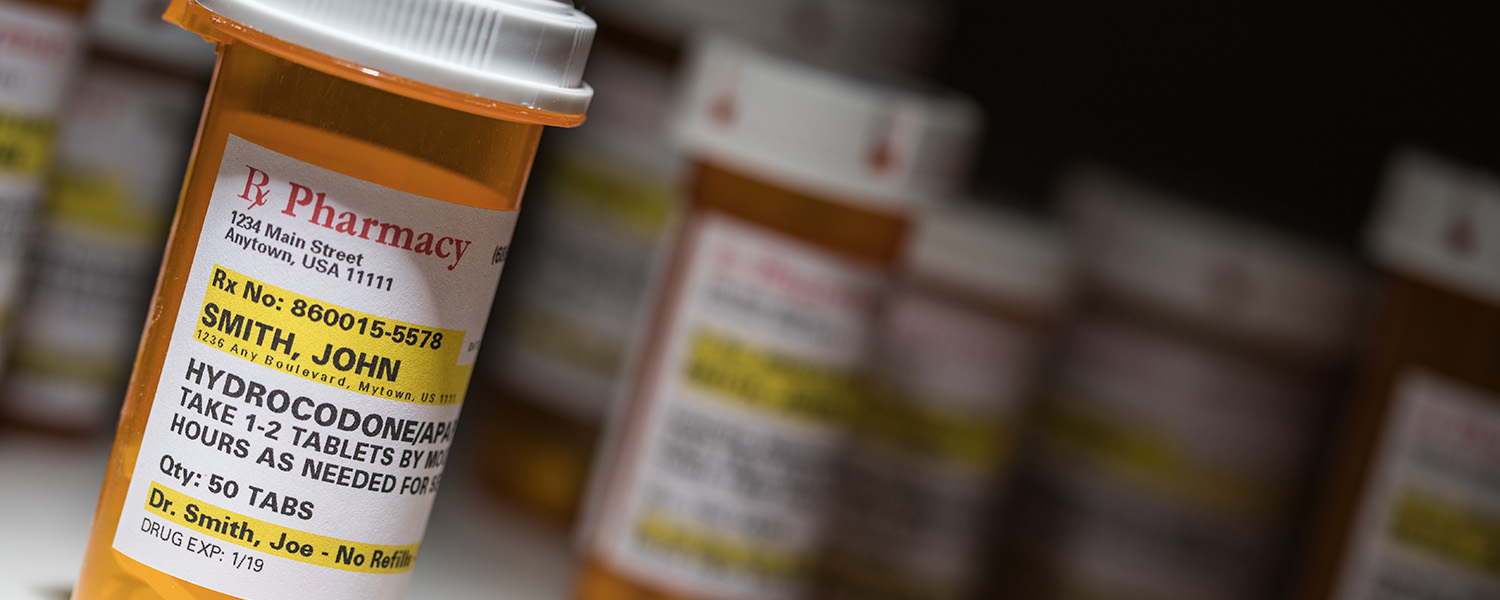
FDA OKs Generic OTC Naloxone Spray Approved by FDA

The FDA has granted approval for Amneal Pharmaceuticals to release a generic 4 mg naloxone hydrochloride nasal spray for over-the-counter (OTC) use, an announcement made in a press release.
This approval expands the options available for naloxone hydrochloride nasal sprays for OTC use in the United States to three. Naloxone hydrochloride is an opioid antagonist designed to temporarily reverse opioid drug overdoses, including those caused by heroin, fentanyl, and prescription opioids. Its mechanism involves blocking the effects of opioids, thereby restoring normal breathing within 1 to 2 minutes.
The significance of this approval is underscored by the opioid epidemic gripping the United States. Overdose deaths, particularly involving opioids, have surged in recent years. Data from the National Institute on Drug Abuse revealed over 106,000 overdose deaths in 2021, with more than 80,000 involving opioids. The situation continued to escalate in 2022, with over 109,000 overdose deaths, primarily attributed to synthetic opioids like fentanyl.
The approval of Amneal's generic naloxone hydrochloride nasal spray follows the FDA's earlier approval of OTC Narcan in March 2023. Narcan, manufactured by Emergent BioSolutions, paved the way for the transition of naloxone hydrochloride from prescription to OTC status. The FDA's decision was informed by data demonstrating the safe and effective use of the drug without the direct supervision of healthcare professionals.
Acknowledging the urgency of the overdose crisis, the FDA reiterated its commitment to facilitating access to naloxone products. The approval of OTC naloxone aligns with the agency's efforts to address the complexities of the overdose crisis and improve access to life-saving treatments.
Following Narcan's approval, Harm Reduction Therapeutics obtained OTC approval for its 3 mg naloxone hydrochloride nasal spray, RiVive, in July 2023. The company aims to distribute its product across the United States either free of charge or at a low breakeven cost, further enhancing access to emergency overdose treatment.
Amneal's launch of its generic naloxone hydrochloride nasal spray reflects its dedication to addressing the public health emergency. The company aims to provide affordable access to naloxone nasal spray without the need for a prescription. By making this emergency treatment readily available, Amneal hopes to empower individuals to save lives and mitigate the devastating impact of opioid overdoses on families and communities.
In conclusion, the approval of Amneal's generic naloxone hydrochloride nasal spray for OTC use represents a significant milestone in combating the opioid epidemic. With multiple options now available, there is greater potential to expand access to life-saving treatments and alleviate the burden of opioid-related overdose deaths in the United States.
McKinsey Under US Opioid Probe
McKinsey & Co. faces a criminal investigation in the United States concerning its alleged involvement in exacerbating the opioid epidemic.
Federal prosecutors are focusing on the consulting firm's advising of OxyContin maker Purdue Pharma and other drug companies, suspecting McKinsey of contributing to the marketing strategies that promoted the sales of prescription painkillers, leading to widespread addiction and fatal overdoses.
Both McKinsey and the U.S. Justice Department declined to comment on the matter. The investigation revolves around whether McKinsey engaged in a criminal conspiracy by advising Purdue and other pharmaceutical companies on marketing tactics to increase sales of opioids. Additionally, prosecutors are probing whether McKinsey participated in healthcare fraud, as its consulting work allegedly resulted in fraudulent claims to government programs like Medicare.
Moreover, investigators are examining whether McKinsey obstructed justice. This aspect of the inquiry pertains to McKinsey's acknowledgment of terminating two partners who discussed deleting documents related to their work on opioids. The probe, initiated several years ago, involves Justice Department officials from offices in Washington, Massachusetts, and Virginia. Both parties are engaged in discussions to resolve the investigation.
It's essential to note that investigations do not imply wrongdoing, and officials conducting the inquiry may pursue criminal charges, seek civil sanctions, or close the probe without further action. The scrutiny of McKinsey's past involvement with drugmakers underscores the enduring repercussions of its consulting work on opioids. The potential for criminal charges against the company or its executives, as well as substantial financial penalties, intensifies the stakes of this investigation.
Previously, McKinsey settled multiple opioid-related lawsuits with agreements totaling nearly $1 billion. These settlements addressed legal actions brought by all 50 states, Washington, D.C., U.S. territories, local governments, school districts, Native American tribes, and health insurers. In 2019, McKinsey announced it would cease advising clients on opioid-related businesses. None of the settlements involved admissions of liability or wrongdoing.
In response to inquiries, Purdue Pharma did not immediately provide a comment. The company pleaded guilty in 2020 to criminal charges related to its handling of opioid painkillers. Following its bankruptcy filing in 2019, Purdue negotiated a settlement valued at approximately $10 billion to resolve numerous lawsuits alleging its contribution to the opioid epidemic. However, the Supreme Court intervened in the settlement process, and a ruling on a Biden administration challenge to the deal is imminent.
Prosecutors have yet to make any charging decisions in the criminal investigation of McKinsey. The complexity of the case involves thorough examination of voluminous documents and ongoing discussions with McKinsey's legal representatives.
Philadelphia Settles $110M Opioid Suit with Walgreens
Walgreens, a pharmacy giant, has agreed to pay Philadelphia $110 million in a settlement concerning the city’s opioid addiction crisis.
This settlement is set to support the Kensington Community Revitalization Plan and other initiatives aimed at enhancing public health and safety, particularly in neighborhoods severely affected by the opioid crisis. The city had filed a lawsuit against Walgreens in 2021, alleging its role in fueling the epidemic.
The agreement outlines that Philadelphia will receive the funds over a five-year period, with the intention of addressing the harm inflicted by the crisis through substance use education, treatment, prevention, and community engagement efforts. Philadelphia’s City Solicitor emphasized the significance of holding entities accountable for perpetuating harm in communities and expressed hope that the settlement funds will expedite efforts to combat addiction and prevent further loss of life. The initial portion of the settlement is expected to be received in September.
Philadelphia initiated legal actions against opioid manufacturers, distributors, and pharmacies in 2017, aiming to hold them responsible for their contributions to the crisis. The establishment of the Opioid Settlement Fund in 2023 aimed to manage and distribute funds obtained from various lawsuits related to the epidemic.
Earlier, pharmacy giants CVS, Walmart, and Walgreens urged Ohio's top court to dismiss a $650.9 million judgment against them for their alleged role in fueling the opioid crisis. They argued over the Ohio law bars claims of public nuisance brought by Lake and Trumbull counties.
The lawsuit is part of broader litigation nationwide and accuses the pharmacies of exacerbating the epidemic. Although they settled other cases, CVS, Walmart, and Walgreens contested this verdict, prompting the 6th U.S. Circuit Court of Appeals to seek clarification from the Ohio Supreme Court on unresolved legal issues. The counties maintain that the law doesn't prevent them from seeking relief to address future harm.

