What Happened In The MassTorts World Last Month? 2017-Dec
Plaintiffs' Request To Reinstate 13 Risperdal Cases
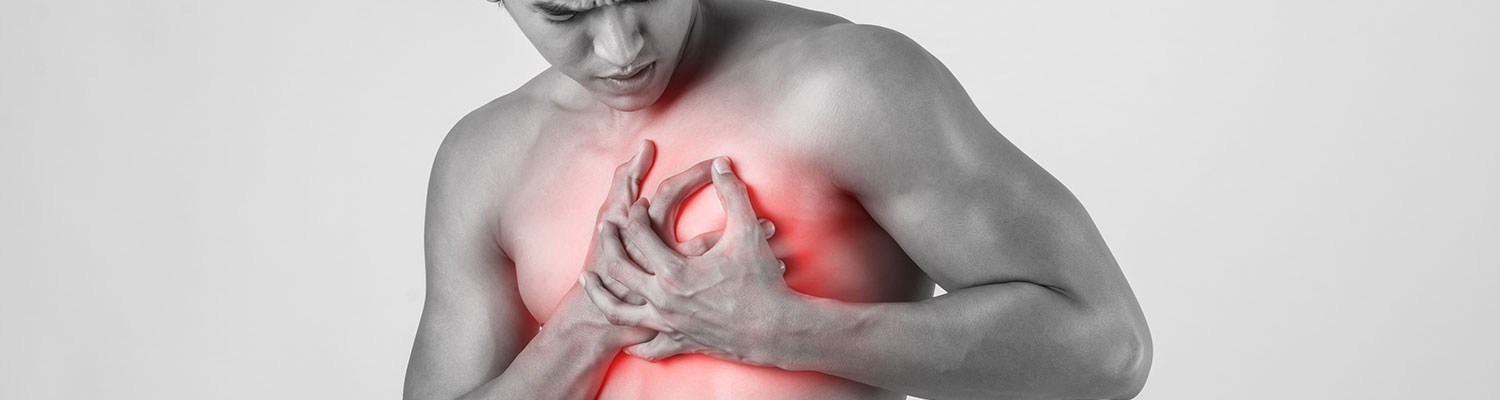
Plaintiffs' request to reinstate 13 Risperdal gynecomastia cases was denied by a Pennsylvania state appeals panel agreeing with the trial judge that the claims were preempted by Michigan's drug shield law and that the plaintiffs could not prove that the fraud exception applied to their claims. Janssen Pharmaceuticals Inc. is the defendant named in this litigation.
Abilify Defense Requests access to Plaintiffs' Computers
In an attempt to collect more evidence of undocumented online gambling, Bristol-Myers Squibb and Otsuka Pharmaceuticals defense attorneys have put forth a request before all the federal courts presiding over the Abilify gambling lawsuits to grant them access to the plaintiffs\’ computers to prove that the alleged compulsive behaviors may have existed before or after the consumers began using the medication.
The plaintiffs have opposed this request calling it 'the most intrusive invasion of privacy in today's digital age,' pointing out that there are other 'less intrusive' ways of obtaining sensitive information.
More than 400 Abilify product liability lawsuits are filed nationwide under MDL No. 2734 (in Re: Abilify Compulsive Behavior Products Liability Litigation), centralized in October 2016, presided by Chief Judge M. Casey Rodgers in the U.S. District Court for the Northern District of Florida. Allegations include compulsive behaviors, such as uncontrollable gambling, shopping, sexual activity or other destructive actions as well as failure to warn the consumers and the medical community about the known link between Abilify and gambling, which may have allowed the users to monitor for signs that may emerge shortly after starting the use of the drug. Recent developments indicate that the first bellwether trials may be delayed from their originally scheduled start in June 2018.
FDA pressurized to issue a Benicar recall

Public Citizen, a prominent consumer watchdog group, filed a recall petition for the FDA to issue a Benicar recall and ban all the other olmesartan drugs, such as Benicar HCT, Tribenzor, and Azor stating these have been linked to the development of sprue-like enteropathy causing severe diarrhea and weight loss that may surface months or even years after the first use of the drug, endangering the patients with unnecessary risks. The group highlighted the fact that the use of the active ingredient olmesartan, outweighs any potential benefits provided to the consumers, stating the use can cause permanent damage to the intestines known as villous atrophy from Benicar. Daiichi Sankyo and Forest Laboratories who faced more than 2,000 Benicar lawsuits together announced a settlement amount of $300 million in mid-2017 for those who suffered chronic diarrhea and other gastrointestinal injuries after using Benicar, Azor, and Tribenzor without accepting any liability.
Global Settlement announced before Axiron Bellwether Trial Begins

Axiron bellwether trial dates, scheduled to begin on January 29 and March 5 this year, were vacated by the federal court after being informed by the counsels of the respective parties that a global settlement of all the claims was decided. The court further granted 45 days for the parties to finalize a Master Settlement Agreement.
Eli Lilly and Company, the maker of Axiron is one of the defendants of over 6,000 centralized testosterone replacement therapy (TRT) lawsuits belonging to MDL No. 2545 in the U.S. District Court for the Northern District of Illinois under U.S. District Judge Matthew Kennelly. Three bellwethers convened last year resulted in two wins for the plaintiffs totalling about $290 million to be paid by AndroGel maker AbbVie and one win for the Testim gel defendant Auxilium. Plaintiff attorneys are fighting for compensation on behalf of individuals who suffered a heart attack, stroke, blood clot or other injuries.
The court further released case management orders for the parties involved to prepare additional 82 cases to be ready for trial over the next two years. The first wave of 12 cases was to be selected by December 21 for August 1, 2018. The second wave of 24 cases is to be selected by February 1, 2018, and a third wave of 46 cases is to be selected by April 2, 2018. Additional waves are expected to be trial ready by January 1 and July 1 of 2019, respectively.
Threatening letters received by Scientists

A number of international scientists who were part of the World Health Organization's International Agency for Research on Cancer (IARC) team involved in the independent research about the potential cancer risks associated with Roundup claim they received what can be termed as 'threatening letters' from Monsanto, the manufacturer of Roundup. The scientists did not take well to the request, with many firing back at Monsanto, calling the company out on what they saw as veiled threats. This came after IARC classified 'glyphosate', the active ingredient in Roundup as a probable human carcinogen. According to our sources, the letters politely ordered them to turn over all of their files or preserve them for pending legal action in the U.S. court. However, the company did not send those letters to U.S. scientists who worked with the IARC. Roundup has been linked to causing non-Hodgkin lymphoma following exposure to Roundup by farmers, landscapers, and other individuals. The recently formed Roundup MDL No. 2741 is presided by U.S. District Judge Vince Chhabria and the first trial in the Roundup litigation is set for June 18, 2018, in the Superior Court for the County of San Francisco. Plaintiffs allege that they or their decedents developed non-Hodgkin's lymphoma after using Roundup over the course of several or more years. Monsanto continues to claim that the product is safe for individual consumers and encourages its use by homeowners.
The first win for 'Xarelto Plaintiff'

The first Philadelphia Xarelto bellwether trial, convened in the Philadelphia's Complex Litigation Center, not only went in favor of the plaintiff but also was the first win for a 'Xarelto Plaintiff' after three straight losses in the federal court. In response to the plaintiff's allegations that Xarelto caused severe bleeding after being prescribed by her doctor and inadequate warnings provided by the drug makers, the state court jury awarded $29 million in damages against the defendants Johnson & Johnson and Bayer AG.
Xarelto lawsuits are centralized in the U.S. District Court for the Eastern District of Louisiana before Judge Eldon E. Fallon as a part of MDL- 2592 Xarelto Products Liability Litigation. Currently, more than 22,000 Xarelto cases are pending in several courts across the country. With the first win proving that a Xarelto prescription caused internal bleeding and no adequate warnings provided by the manufacturers, preparation will begin for the remaining Xarelto cases.
Opioid MDL Approved; Purdue May Opt Global Settlement

On December 5, 2017, a formal nod came in for the formation of Opioid MDL: 2804 by the U.S. Judicial Panel on Multidistrict Litigation. To promote the just and efficient conduct of the litigation and for the convenience of the parties and witnesses, the panel announced centralization in the Northern District of Ohio. Plaintiffs include cities, counties, and states alleging that the manufacturers of prescription opioid medications overstated the benefits and downplayed the risks of the use of their opioids and aggressively marketed (directly and through key opinion leaders) these drugs to physicians; and the distributors failed to monitor, detect, investigate, refuse, and report suspicious orders of prescription opiates.
Judge Daniel Polster is appointed to oversee the litigation related to manufacturing, marketing, distribution and sales practices of prescription opioid drugs by drug makers and distributors.
More than a dozen states and about 100 counties and cities have already sued Purdue Pharma LLP and other opioid makers and drug distributors. News of a global settlement is indicated by Purdue that may resolve state investigations and cases.
With the recent MDL formation, it has been reported that the FDA issued the lowest number of warning letters to pharmaceutical companies caught lying about their products while on the other side the US Department of Justice launched a massive crackdown on opiate drug makers.
Apart from the lawsuits filed in the Opioid MDL, separate investigative subpoenas including demand letters and other requests have been served to eight companies by Texas and coalition of 40 other states. The manufacturers and distributors named are Endo Pharmaceuticals, Janssen Pharmaceuticals, Teva Pharmaceuticals' Cephalon, Allergan and their related entities, Purdue Pharma, etc.
Plaintiffs Now Opposing Centralization of Stockert 3T Cases

Abilify (aripiprazole) is an atypical antipsychotic developed by Otsuka Pharmaceutical Co., Ltd. In 1999, Otsuka partnered with Bristol-Myers Squibb (BMS) to complete development, obtain approvals, and market aripiprazole in the U.S. In November 2002, it was approved by the FDA for the treatment of schizophrenia in adults. In October 2007, the FDA approved Abilify for the treatment of schizophrenia in adolescents aged 13-17 years. In 2004, it was approved to treat symptoms related to Bipolar I Disorder in adults. Later in 2008, it was approved to treat patients 10 to 17 years old. This was followed by an approval for the treatment of unipolar depression as an adjunct with an antidepressant medication in November 2007. In 2009, it was approved to treat irritability associated with an autistic disorder for patients 6 to 17 years old. Abilify is not approved for the treatment of dementia-related psychosis. Elderly patients treated with antipsychotic drugs for dementia-related psychosis are at an increased risk of death.
Aripiprazole is used predominantly for the treatment of schizophrenia and bipolar disorder but can also be used as an add-on treatment for major depressive disorder, obsessive-compulsive disorder (OCD), tic disorders, and irritability associated with autism.
Abilify is known to decrease hallucinations and improve concentration. When there is a hormonal imbalance, Abilify either increases or decreases the levels of dopamine or serotonin in the brain. The drug arbitrates its action by either blocking receptors or by binding to them and inducing an opposite response to the receptor's normal role. A large number of patients treated with Abilify were reported to develop the habit of compulsive gambling and got racked up with large amounts of debts. Other pathological behaviors like excessive eating, excessive shopping, and Hypersexuality are also noted who started using or increased dosage of Abilify.
Young patients with depression and elderly patients with dementia consuming Abilify are found to be at a high risk of suicidal thoughts or actions. It also increases the risk of developing diabetes.
In 2013, the marketing rights were returned to Otsuka by BMS; however, BMS kept manufacturing the drug.
In November 2017, the FDA approved Abilify MyCite, a digital pill containing a sensor intended to record when its consumer takes their medication.
Some Facts As Stated By The FDA:
- Aripiprazole is available under the brand names Abilify, Abilify Maintena, Aristada, and also as generics.
- Common side effects of aripiprazole include dizziness, lightheadedness, drowsiness, excess saliva/drooling, blurred vision, weight gain, constipation, feeling the urge to move constantly, and trouble sleeping.
- In 2015, approximately 7.7 million prescriptions for oral aripiprazole were dispensed and approximately 1.6 million patients received a dispensed prescription for oral aripiprazole from U.S. outpatient retail pharmacies.
Open configuration options
Open configuration options
SERIOUS ALLEGED INJURIES MAY INCLUDE:
- Pathological Gambling
- Binge Eating
- Hypersexuality
- Dementia Suicide Risk
FDA SAFETY WARNINGS:
In 2004, the FDA required a boxed (“black box”) warning to be added to package inserts for antidepressants in order to call attention to an increased risk of suicidal thoughts and behavior (suicidality) in children and adolescents taking these drugs. In 2007, the FDA extended the age range covered by the warning to include young adults up to 24 years of age.
In 2006- For Abilify, the black box warning indicated that the drug increases the risk of death by stroke in elderly patients with psychosis related to dementia. The FDA did not recommend this medication for elderly people who had dementia.
On May 3, 2016, the FDA warned that compulsive or uncontrollable urges to gamble, binge eat, shop, and have sex had been reported with the use of the antipsychotic drug aripiprazole.
LEGAL UPDATES:
Defendants: Otsuka Pharmaceutical Co., Ltd., Bristol-Myers Squibb (BMS), & Otsuka America Pharmaceutical, Inc. (OAPI).
Defendant Law Firm:
Otsuka is represented by Matthew A. Campbell, Rand K. Brothers and Luke A. Connelly of Winston & Strawn LLC and Hal K. Litchford and Kelly Overstreet Johnson of Baker Donelson Bearman Caldwell & Berkowitz PC.
Bristol-Myers is represented by Larry Hill and Charles F. Beall Jr. of Moore Hill & Westmoreland PA; Anand Agneshwar, Matthew Eisenstein and Paige H. Sharpe of Arnold & Porter; and Lauren S. Colton of Hogan Lovells.
Defendants’ Liaison Counsel
Larry Hill - Moore, Hill & Westmoreland, P.A.
Allegations: Allegations include Abilify harmed patients by causing uncontrollable urges, resulting in financial, psychological, and physical damages. The defendants failed to adequately study the drug and its possible side effects. The defendants knew – or should have known – that Abilify causes or contributes to compulsive behaviors. The defendants spent millions of dollars on misleading advertising that overstated the drug’s benefits and understated the risks and made payments to physicians to promote Abilify. The benefits of using the product do not outweigh the risks.
Plaintiff Steering Committee appointed by US District Judge M. Casey Rodgers
Behram V. Parekh - Kirtland & Packard LLP.
Chris T. Hellums - Pittman, Dutton & Hellums, P.C.
George T. Williamson, Jennifer R. Liakos - Napoli Shkolnik PLLC.
M. Brandon Smith, Esq. - Childers, Schlueter & Smith L.L.C.
Marlene J. Goldenberg - GoldenbergLaw, PLLC.
Shanon J. Carson - Berger & Montague, P.C.
Lawsuit Status:
October 2016: Abilify lawsuits are consolidated under MDL No. 2734, presided by U.S. District Judge M. Casey Rodgers, in the Northern District of Florida. As of November 2018, the court papers show nearly 2,000 pending lawsuits in the Abilify MDL.
May 2018: Abilify New Jersey MCL Designation – Atlantic County for centralized management by Judge Nelson C. Johnson; trials were expected to begin by the end of 2018.
Important Legal Proceedings, Verdicts & Settlements:
April 2018: The defendants settled three cases representing nearly 800 plus centralized lawsuits for an undisclosed amount of days before they were scheduled to go for trial.
May 2018: A global settlement order released by Judge Rodgers stating the defendants had time until September 1, 2018, to determine how they will settle a large number of lawsuits filed against them. Though the parties indicated progress about the settlement negotiations, a final settlement program news had not been submitted to the court.
June 2018: Judge Rodgers indicated that a group of 100 cases to be picked randomly before July 6, 2018, for the second trial pool.
August 2018: Judge Rodgers instructed all plaintiffs in Abilify litigation to furnish more information to estimate the inventory of the alleged gambling cases filed against the drug makers in the federal MDL.
September 2018: Six Abilify cases selected for "Fast-Tracked" discovery.
November 2018: 21 Abilify cases remanded back to their home state courts to allow the litigation to move forward in the state court system while the bellwether trial process continues in the federal courts.
December 2018: The Court announced the discovery schedule for six Abilify gambling cases that were selected for a fast-tracked discovery and trial.
January 2019: Bristol-Myers Squibb Co and Otsuka Pharmaceutical Company requested the judge to toss gambling lawsuits filed by 550 plaintiffs unless they furnish more information linked to their use and past medical records.
February 2019: Bristol-Myers Squibb Co. and Otsuka Pharmaceutical Co. Ltd. reached a confidential global settlement with thousands of plaintiffs who alleged that the antipsychotic drug causes uncontrollable gambling urges.
September 2019: U.S. District Judge M. Casey Rodgers dismissed 149 cases linked to the side effects of the antipsychotic drug due to plaintiffs' failure to comply with court orders following the announcement of a global settlement by the defendants Otsuka Pharmaceutical Co. Ltd. and Bristol-Myers Squibb Co. in February 2019.
EVIDENCE:
- Usage In Pharmacy Records
- Duration Of Usage
- Indication Of Usage In Medical Records
Open configuration options
Open configuration options
Medical Record Review and claim validation of Abilify case should take approximately 3 hours in most instances; however, this approximation may vary in cases based on the volume of records.
Another Onglyza case filed

A product liability lawsuit was filed against Bristol-Myers Squibb and AstraZeneca by a woman who suffered heart failure and other severe cardiovascular complications alleging to be side effects of the saxagliptin-based diabetes drugs, Onglyza and Kombiglyze XR. The complaint was filed in the U.S. District Court for the Northern District of Alabama in early December. The lawsuits state that the defendants had knowledge that there was a significantly increased risk of adverse events associated with Saxagliptin, despite this they continued to manufacture, market, distribute, sell, and profit from the sales of Saxagliptin. A request for consolidating Onglyza lawsuits was made recently. The U.S. JPML is expected to consider oral arguments on the motion during an upcoming hearing in January 2018.
Out-of-State Vaginal Mesh & Talcum Cases Allowed to Proceed

A judge did not allow the Supreme Court June 2017 Bristol-Meyer Plavix ruling influence his decision and allowed the out-of-town plaintiffs from proceeding in state court vaginal mesh and talc powder lawsuits. In June 2017, the U.S. Supreme Court determined that lawsuits could not be pursued against the maker of the blood thinner Plavix in states where the plaintiff did not purchase or consume the product unless the company is incorporated or headquartered there.
This ruling affected the fate of many lawsuits which were filed out-of-state. However, a Philadelphia Court of Common Pleas Judge only agreed to dismiss one out-of-state plaintiff’s case out of more than 100 transvaginal mesh claims pending against Johnson & Johnson's Ethicon subsidiary in the state court’s mass tort program. This was based on the finding that the company that manufactured the actual mesh implanted in the out-of-state plaintiffs had sufficient ties to Pennsylvania.
A Missouri judge too upheld a $110 million talcum powder cancer verdict awarded to an out-of-state resident, finding the defendants in the case engaged in sufficient acts during the manufacturing, labelling, and sale of Johnson's Baby Powder and Shower-to-Shower products in Missouri to establish the jurisdiction in the state court system.
Growing MDLs and Lawsuits

The proton-pump inhibitor product liability litigation MDL No. 2757 is witnessing a steady rise in the number of cases filed against the makers alleging they provided false and misleading information about the safety of the drugs for years, risking the lives of thousands who faced an increased risk of acute kidney injury, chronic kidney disease, kidney failure, and other renal problems after using their products. As per our sources, about 370 lawsuits are pending in the federal court system presided over by Judge Claire C. Cecchi. Additionally, 127 complaints are filed in the Delaware Superior Court and one case filed in Missouri Circuit Court that involves 28 different plaintiffs.
MDL 2750, the federal litigation of Invokana and Invokamet lawsuits is growing steadily as plaintiffs around the country continue to file lawsuits against the drugmaker Janssen Pharmaceuticals, Inc. for suffering serious complications alleged to be a result of their exposure to canagliflozin, the active ingredient present in these drugs. The cases were centralized in December 2016 before Judge Brian R. Martinotti, in the U.S. District Court, District of New Jersey and currently has 952 cases pending.
A Louisiana woman filed a product liability complaint in the U.S. District Court for the Eastern District of Louisiana against Bayer for manufacturing and selling Essure birth control device claiming it to be dangerous and putting her life at a serious risk. This was after she developed severe complications from the Essure coils resulting in the need for an additional surgery to remove the device, further leading to health problems. The woman also complained of suffering from heavy bleeding with large clots, severe abdominal cramping, pelvic pain, stomach bloating, leg pain, heavy menstrual cycles, severe itching, headaches, and fatigue.
The device has also been linked to requiring a necessary hysterectomy. The manufacturer Bayer has reported in their 2016 financial report of losing roughly $413 million USD for Essure and revealed that nearly 3,700 patients have filed lawsuits against them as of January 2017 for Essure complications.
Thousands of Essure lawsuits are filed nationwide. In 2016, Judge Winifred Y. Smith, coordinated 55 cases before a single judge in Alameda County Superior Court under Judicial Council Coordination Proceeding No. 4887. Apart from this, several groups of Essure cases have been consolidated in other courts. At least 18 federal lawsuits are currently consolidated in the U.S. District Court for the Eastern District of Pennsylvania as of November 2016. However, Essure lawsuits are filed individually by each plaintiff and are not class actions.
A North Carolina woman filed a lawsuit against AstraZeneca and Takeda Pharmaceuticals in the U.S. District Court for the District of New Jersey alleging she became a case of chronic kidney disease following the long-term use of Nexium and Dexilant, and the drug makers failed to adequately warn consumers and the medical community about the potential kidney risks. Nexium lawsuits are centralized in MDL No. 2757 under Judge Claire C. Cecchi in the U.S. District of New Jersey.
Multiple revision surgeries and permanent injuries by Bard Monofilament Knitted Polypropylene Mesh used during an incisional hernia repair in January 2006 were the reasons why a lawsuit was filed by a man against C.R. Bard and its Davol subsidiary in the U.S. District Court for the Eastern District of Louisiana.
Nearly 350 Pelvic Mesh Suits Dismissed

With Boston Scientific settling nearly 350 pelvic mesh lawsuits, a West Virginia federal judge, Judge Joseph R. Goodwin, dismissed the settled suits filed against the company under the multidistrict litigation 2326 in the U.S. District Court for the Southern District of West Virginia. Allegations were filed against the company for making defective pelvic mesh implants. The settlement was reached almost 5 years after the MDL was formed. The parties had filed two joint motions for dismissal with prejudice. The case involves seven MDLs composed of 28,000 individual transvaginal mesh cases against Boston Scientific and other makers of mesh implants, such as American Medical Systems, C.R. Bard Inc., and Johnson & Johnson.
100's of Byetta, Victoza, Januvia & Janumet Cases Reinstated

At least 700 Byetta lawsuits, Januvia, Janumet, and Victoza lawsuits, belonging to MDL No. 2452 were reinstated through a federal court appeal made by plaintiffs who allege they developed pancreatic cancer following the use of the diabetes drugs made by these manufacturers.
In November 2015, Judge Battaglia granted a summary judgment in favor of the defendants following a preemption protocol, dismissing all the cases which were a part of MDL No. 2452. Following this, in September 2016, the plaintiff attorneys filed an appeal to the Federal Appeals Court stating that Judge Battaglia misinterpreted the U.S. Supreme Court preemption law, responding to which the court ruled in favor of the plaintiffs. The court also overturned a discovery decision which had denied the plaintiffs' requests for documents from the drug makers stating it was unduly burdensome and also allowed some of the previously denied testimony by one of the plaintiffs' expert witnesses.
The litigation will continue to be presided over by Judge Battaglia for further proceedings. The announcement of bellwether trials is expected soon.
Jury Awards $15 Million to a Pelvic Mesh Injury Victim

A Bergen County Jury of New Jersey unanimously awarded nearly $15 million to a woman who alleged she suffered from debilitating pain caused by one of the two pelvic mesh devices that she received in 2008 sold under J&J’s Ethicon brand name Prolift. The verdict supported the claim that the manufacturer failed to adequately warn about the potential side effects.
Seven pelvic mesh manufacturers are facing thousands of lawsuits under individual MDLs consolidated by U.S. District Judge Joseph R. Goodwin, in early 2012, in the U.S. District Court for the Southern District of West Virginia. The total number of cases under the umbrella was over 102,133 cases as of March 2017. Formation of MDL No. 2327 in Re Ethicon, Inc., Pelvic Repair System Products Liability Litigation was a part of the consolidation. Ethicon has consistently had the largest number of product liability cases filed in the federal court with about 38,035 cases filed and 5,547 cases closed. These cases all relate to the use of transvaginal surgical mesh to treat pelvic organ prolapse ("POP") and/or stress urinary incontinence ("SUI").
Ethicon released a statement minutes after the verdict saying that it would appeal.
Stryker LFIT V40 Femoral Head Trials

The Stryker LFit v40 lawsuits centralized along with other hip replacement lawsuits before U.S. District Judge Indira Talwani in the U.S. District Court for the District of Massachusetts in April 2017 as a part of multidistrict litigation No. 2768 are in the pre-trial discovery phase and are maintaining a steady pace.
Recently Judge Talwani called on the plaintiffs and defendants to select nine cases each, for a total of 18, to be a part of the initial bellwether discovery pool on or before February 2, 2018. Cases have to be selected on the basis that they were filed on or before December 12, 2017, and a "substantially completed" Plaintiffs' Fact Sheet has been served by the plaintiff on or before January 12, 2018. The judge also informed the parties to submit proposals regarding how many of the 18 bellwether cases will be eligible for the initial trial pool in September 2018. The first set of cases is expected to go before a jury on September 16, 2019.
The Stryker LFit v40 was removed from the market when reports involving complications and failures including taper lock problems came forth. The complaints received pointed that the metals used, a combination of cobalt-chromium and titanium alloy could lead to fretting and corrosion due to the friction placed upon them when the patient's hip joint moved, resulting in pain, inflammation, loss of mobility, disassociation, and the need for a risky revision surgery. Plaintiffs allege the company knew the device was dangerous and failed to ensure the implants worked together safely and properly with the other components. The final bellwether trial cases will be selected by October 5, 2018.
Reviewing a Dismissed Zoloft Birth Defect Case Denied by PA Court
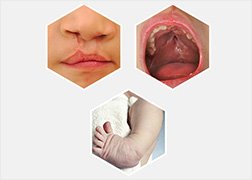
A petition to review a Zoloft birth defect case dismissed earlier when the plaintiffs’ causation expert was excluded by the trial court was denied by the Pennsylvania Supreme Court. The lawsuit was filed by a woman who alleged that her son was born with a giant omphalocele caused by Zoloft and paroxetine consumption during her pregnancy.
No New Trial for the 3rd Xarelto Bellwether Plaintiff

The plaintiff of the 3rd Xarelto bellwether trial, which went in favor of the Xarelto defendants J&J's Janssen Pharmaceuticals and Bayer in August 2017, faced a defeat again when a Louisiana federal judge turned down the request for a new trial.
The plaintiff had planned for a retrial based on the recent scientific study that she hoped could change the jury's mind. Judge Eldon E. Fallon denied her request stating that the newly discovered evidence was actually cumulative of the previously known and admitted evidence.
Xarelto lawsuits are consolidated under MDL No.- 2592 in the U.S. District Court for the Eastern District of Louisiana; nearly 22,000 cases are a part of this MDL while more than 1,500 cases are pending in Philadelphia's Complex Litigation Center.

