What Happened In The MassTorts World Last Week? 2018-May-28
Talcum Mesothelioma Lawsuit Underway in South Carolina

Johnson & Johnson, Imery’s Talc America, and the Rite Aid drug store chain are the defendants named in the Talcum Powder lawsuit that went to trial on May 14 in South Carolina’s Darlington County Court of Common Pleas. The lawsuit was filed by the family of Bertila Boyd-Bostic and claims that the talc present as an ingredient in J&J's Baby Powder contained asbestos and the life-long use of this brand of talcum by Bertila Boyd-Bostic led to her diagnosis of pericardial mesothelioma in 2016 and eventually to her death in 2017. The lawsuit claims that the defendants have known for decades that the Talcum Powder was contaminated with asbestos and they purposely concealed that information and failed to warn the consumers.
More than 6,600 talcum powder ovarian lawsuits are filed on behalf of women alleging that the long-term use of talc products for maintaining feminine hygiene caused ovarian cancer. Since February 2016, $55 million to $417 million verdict amounts have been awarded to plaintiffs in Johnson & Johnson talcum powder ovarian litigation.
Bard IVC Filter Lawsuit: Motion Filed For A New Trial
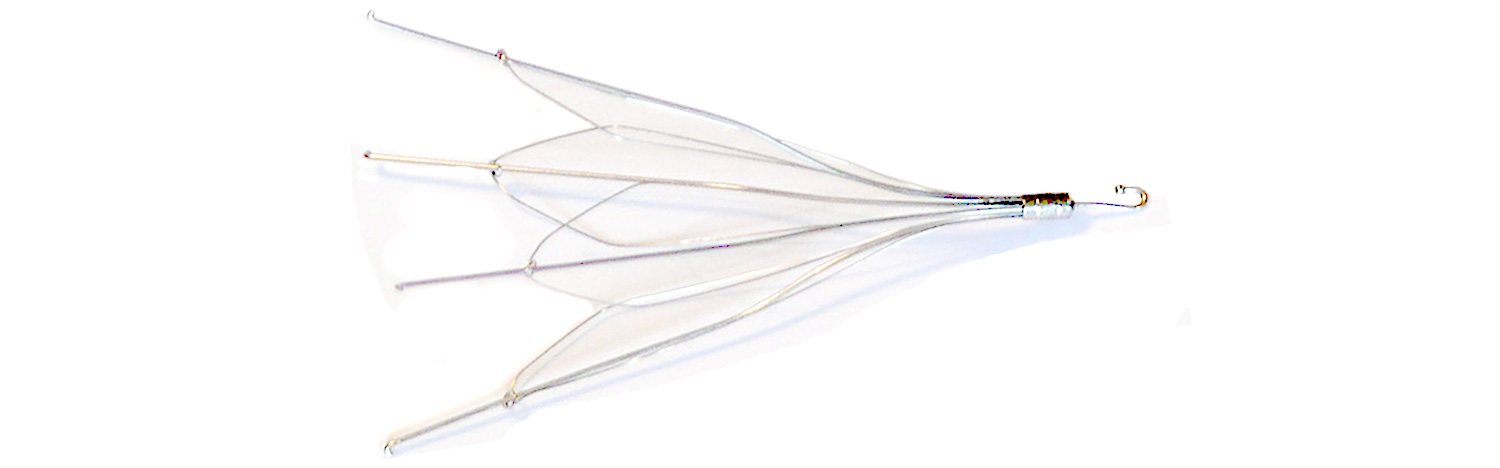
C.R. Bard filed a motion on April 23, 2018, seeking a new trial after Plaintiff Sherri B was rewarded $4 million in the March IVC Filter bellwether trial by the court presided by U.S. District Judge David Campbell in the District of Arizona. In the statement filed, Bard insisted that the plaintiff “utterly failed to present any evidence at the trial on the fundamental premise” of negligent failure to warn and called the jury's verdict as "irreconcilably inconsistent”. The company filed a motion for judgment as a matter of law to turn the verdict in their favor.
Sherri had the Bard G2 Filter implanted in 2009 and had to undergo an open heart surgery to remove the broken pieces of the device that got deposited in her heart and spine. The jury did not find Bard guilty for product liability but stated that the company was liable for not warning the doctors about the possible adverse effects.
This lawsuit is a part of the IVC Filter MDL No. 2641 (in re: Bard IVC Filters Products Liability Litigation) established in the U.S. District Court for the District of Arizona. More than 3,000 IVC Filter lawsuits are pending in the consolidated action against C.R. Bard for its Recovery, G2, G2 Express, G2X, Eclipse, Meridian, and Denali IVC Filters, over claims that due to the manufacturing and design defects, Bard filters are more dangerous than other IVC filters and that Bard failed to warn about the higher risks.
Plaintiff & Defendant Fact Sheets for Ethicon Physiomesh MDL
Observing the growing number of Ethicon Hernia Mesh complaints, the Federal Court decided to adopt the Plaintiff and Defendant Fact Sheets, last week, for use in cases selected for the initial discovery pool pursuant to practice and procedure order no. 7.
The Plaintiff Fact Sheet demands the plaintiff to identify key factors related to the adverse events linked to Physiomesh. The Defendant Fact Sheet needs Ethicon, Inc. and/or Johnson & Johnson to detect information regarding the salesperson who interacted with the health care provider along with sales data.
About 900 Hernia Mesh lawsuits are pending in the Northern District of Georgia as a part of MDL No. 2782 (in re: Ethicon Physiomesh Flexible Composite Hernia Mesh Products Liability Litigation). The lawsuits have a similar assertion that the defective nature of the mesh resulted in serious injuries and complications, other complaints include allegations that the polypropylene used in the device is incompatible with the human tissue and serves a breeding ground for bacteria.
Lawsuit Filed Against C.R. Bard’s Dulex Hernia Mesh
Martha Karen Jones filed a complaint against C.R.Bard in the U.S. District Court for the Eastern District of Tennessee for the infections caused due to the defectively designed Bard Dulex Hernia Mesh. In her statement, Jones indicated that C. R. Bard and its subsidiary Davol Inc. are responsible for hiding critical information regarding the faulty functioning of the mesh, which was implanted in her body during a hernia revision surgery in June 2008. However, the mesh got infected and had to be removed in September 2012 and resulted in her undergoing additional surgeries until May 2017.
The lawsuit indicated that the defendants failed to warn the doctors and patients that the expanded polytetrafluoroethylene (ePTFE) construction of the Dulex hernia mesh was faulty.
Following a growing number of Bard Hernia Mesh lawsuits claiming internal damage to the body, the plaintiffs filed a motion for centralization before the U.S. Judicial Panel on Multidistrict Litigation (JPML). The defendants support this decision provided all Bard hernia mesh products containing polypropylene including the Composix Kugel (CK) Hernia Patch complaints that were excluded from their original motion would be included in this MDL.
A hearing is set for May 31 in Chicago or July 26 in Santa Fe, New Mexico, when oral arguments for the motion of consolidation may be scheduled by the JPML. About 900 Ethicon Physiomesh lawsuits and 30 Atrium C-Qur lawsuits are pending in the federal court system.
16 Zofran Lawsuits to be Picked for Early Bellwether Trials
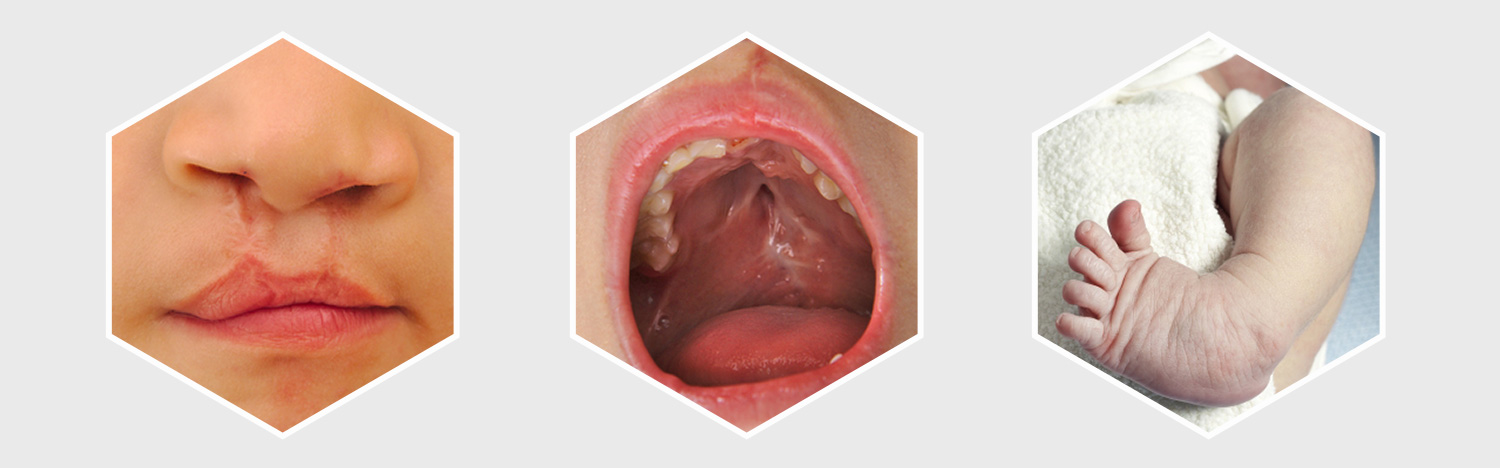
On May 17, 2018, U.S. District Judge Dennis Saylor ordered both parties involved in the Zofran litigation over birth defects to pick a total of 16 cases to proceed to early bellwether trials, assigning half to each side. This would help evaluate the reaction of the jury to certain evidence and testimony. As per the order, the parties have time until June 15 to select the cases.
The order stated that it was mandatory for the cases to include completed fact sheet and all relevant authorizations details submitted to GlaxoSmithKline at least 60 days in advance of case selections. The claims must cover either structural heart damage birth defects, like atrial or ventricular septal defects, or craniofacial birth defects, such as cleft palate and cleft lip. After the lawsuits are selected for bellwether trials, the parties have time till October 31 to discuss the procedures to choose individual cases from that group of lawsuits for future trials.
At present, more than 450 product liability cases are filed against GlaxoSmithKline, over failure to provide timely warning to doctors about the adverse effects of Zofran consumption during pregnancy. The drug was FDA approved to treat nausea in cancer patients, but GlaxoSmithKline took the liberty of marketing it to pregnant women for morning sickness. In October 2015, a multidistrict litigation |LS|MDL No. 2657 In Re: Zofran (Ondansetron) Products Liability Litigation|RS| was created for Zofran birth defects lawsuits in the District of Massachusetts and is presided by Judge Dennis F. Saylor, IV.
In another recent event, GlaxoSmithKline succeeded in getting the federal judge to toss the lawsuits filed by three women over injuries allegedly linked to the generic form of Zofran (also developed by GlaxoSmithKline LLC) from the Zofran multidistrict litigation.
Judge Refuses Defendants’ Plea to Bifurcate PPI Litigation

The plaintiffs and defendants involved in Proton Pump Inhibitors (PPIs) kidney injury litigation submitted contradicting proposals last month on how to proceed with the multidistrict litigation (MDL) witnessing a growing number of lawsuits. While the defendant PPI manufacturers AstraZeneca Pharmaceuticals LP, Procter & Gamble Co., McKesson Corp., Takeda Pharmaceuticals USA Inc., Novartis Pharmaceuticals Corp., and Pfizer Inc. wanted the cases to be handled in two phases, the plaintiffs wanted a fast-track proceeding leading to a bellwether program with trial dates set for early 2020. In a case management order issued by Judge Cecchi on May 18, the defendant’s request was rejected and the parties were ordered to meet and discuss as per the plan proposed by the plaintiffs.
Both the parties are scheduled to discuss how to proceed with the federal MDL in a status conference on May 24. The next status conference is on June 12 for which both parties are required to submit a status report and an agenda by June 8.
The PPI litigation is centralized in the District of New Jersey as MDL No. 2789 (In Re: Proton-Pump Inhibitor Products Liability Litigation |LS|No. II|RS|), presided over by Judge Cecchi. Lawsuits mainly involve Nexium, Prilosec, Protonix, Prevacid drugs that were prescribed to treat heartburn and acid reflux in the stomach. About 4,200 product liability cases are filed in this MDL, each lawsuit alleging that the drug makers failed to warn about the fatal side-effects like kidney failure due to prolonged use of PPI.
Jury Slams J&J with $21.7 Million Verdict in Asbestos Trial

A 68-year old woman was awarded $21.7 million on May 23 by the Los Angeles jury for the lawsuit filed against Johnson & Johnson and its talc supplier Imerys, claiming she was diagnosed with mesothelioma after being exposed to asbestos-laden talcum powder baby powder, which she used on her children and while bowling. The compensatory damages of $21.7 million included 67 % from J&J, and the remaining amount was assigned to other defendants involved in the litigation. As per the plaintiff's lawyer, the jury is unsure about awarding punitive damages to the plaintiff.
The case was filed in 2017. J&J intensely denied the allegations about asbestos contamination in the talcum powder.
This is the second time Johnson & Johnson lost to the plaintiff over a mesothelioma allegation. Earlier in April, a New Jersey Court Jury made J&J and its talc supplier pay $117 million to a man who was detected with mesothelioma. Over 6,600 lawsuits are filed nationwide by women who claimed they developed ovarian cancer after using J&J's Talcum Powder for feminine hygiene purposes.
Zimmer Kinectiv Hip Replacement Case Filed Over Metallosis
On May 19, Judith Harms from Minnesota filed a product liability lawsuit against the manufacturer of Zimmer Kinectiv Hip Replacement System in the U.S. District Court for the District of Minnesota. Plaintiff Harms filed the suit over the development of metallosis, a form of metal blood poisoning due to the defectively designed Hip Replacement System implanted in September 2008 for which she had to undergo a revision surgery in October 2015. In her complaint Harms stated that the head-neck taper junction of the Zimmer Kinectiv corroded and crumbled, leaving metallic debris in the body.
The revision surgery revealed the presence of pericapsular necrotic tissue, thickened capsule, and a brown metallic stained fluid in the area of the hip device. Zimmer's metal head had dislocated from the Zimmer's neck, leaving a black smudge.
Hip Replacement System recalls have spiked in recent years due to the complications arising from its metal-on-metal design. Zimmer is also facing allegations that their Knee Implant System has caused complications for which cases are consolidated as a part of MDL No. 2272 (in Re: Zimmer NexGen Knee Implant Products Liability Litigation) in the U.S. District for New Jersey under Judge Susan D. Wigenton.
In June 2013, MDL No. 2441: Stryker Product Liability Litigation was established in the District of Minnesota, presided by Judge Donovan W. Frank, for Stryker Hip Replacement lawsuits.
Jury Confirms $4 M Punitive Damages for Mesothelioma Victim

Los Angeles jury announced $4 million as punitive damages on May 24, 2018, in favor of Plaintiff Joanne Anderson, who was diagnosed with mesothelioma and awarded $21.7 million as compensatory damages on May 23. The plaintiff alleged she developed mesothelioma due to the exposure to asbestos present in the talc used in J&J’s talcum powder since the 1970s while bowling and using it on her children.
A banker with the same diagnosis was awarded $117 million in punitive and compensatory damages by a New Jersey jury last month. Johnson & Johnson denies the allegation that their Talcum powder contains cancer-causing asbestos. Over 6,600 lawsuits are filed nationwide by women who claim they developed ovarian cancer after using J&J's Talcum Powder for feminine hygiene purpose.

