What Happened In The MassTorts World Last Week? 2020-Oct-12
EMA Re-examines Zantac; Confirms Suspension In The EU

On September 18, the European Medicines Agency (EMA) issued a press release confirming its prior recommendations dated April 3, 2020, to suspend ranitidine throughout the European Union due to the presence of low levels of an impurity called N-nitrosodimethylamine (NDMA). The suspension confirmation is the result of a request made by one of the drug makers selling the generic Zantac to re-examine its decision over the ban.
The recent re-examination of Zantac safety was conducted by EMA’s human medicines committee (CHMP). The committee announced the conditions over lifting the suspension, along with the requirements for companies to provide more data on the possible formation of NDMA from ranitidine inside the body. The CHMP revised the conditions for lifting the suspension for those ranitidine medicines that are given by injection or infusion as a single dose, stating that the NDMA formation in the body is expected to be very low when administered by the stated method.
The Food and Drug Administration (FDA) had issued a similar recall of the heartburn drug from the U.S. market on April 1, 2020, after finding that independent tests showed the levels of NDMA could increase over time and with exposure to high temperatures in ranitidine medications.
The public was not aware of the high levels of NDMA produced by Zantac until the testing was done by an independent online pharmacy, Valisure, in September 2019. The pharmacy claimed that each pill might result in a level of exposure higher than the limit set by the FDA regarding the intake of NDMA and filed a citizen’s petition with the FDA, calling for the recall.
Last month, the FDA also issued guidance recommending the manufacturers of active pharmaceutical ingredients and drug products to implement steps to avoid drug contamination with cancer-causing chemical by-products, such as NDMA and other nitrosamine impurities.
Several lawsuits have been filed in the U.S., after the recall, by former users of Zantac who were diagnosed with bladder cancer, kidney cancer, colorectal cancer, stomach cancer, and other forms of cancer allegedly caused due to years of exposure to NDMA in Zantac.
Sientra Faces Lymphoma Lawsuit Over Textured Breast Implants
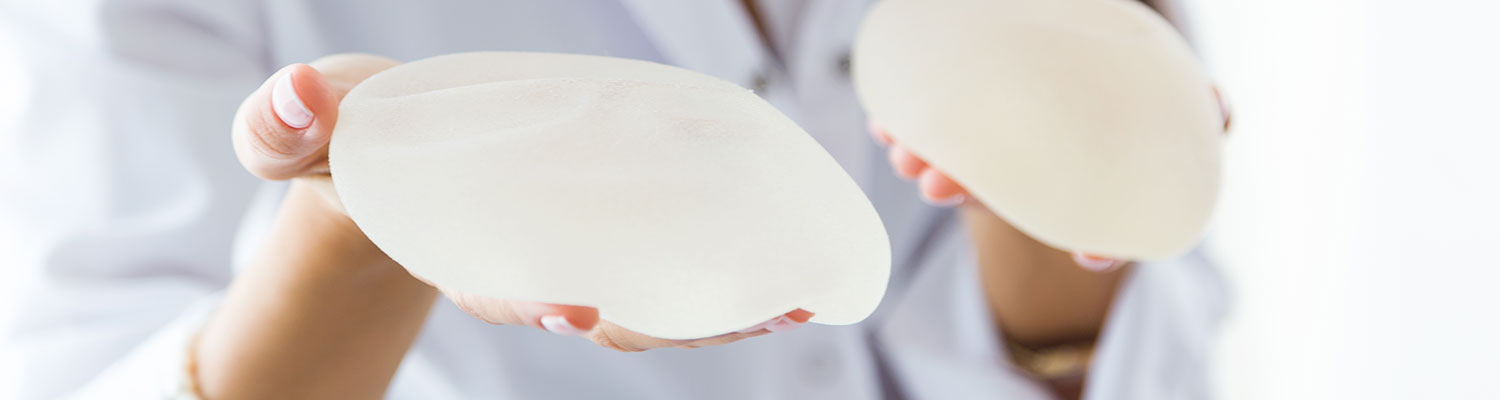
Sientra is facing allegations similar to Allergan over its silicone implants by a Tennessee woman who claims that the textured breast implants made in Brazil resulted in her breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), a rare type of cancer in the surrounding tissue.
The lawsuit was filed by Kelly Painter-Hart and her husband, Seth Hart, in the U.S. District Court for the Eastern District of Tennessee. According to the lawsuit filed, Painter-Hart had bilateral mastectomies in November 2013 as a precautionary measure over breast cancer and was implanted with Sientra textured oval-shaped silicone gel implants.
In September 2019, she went to her surgeon complaining of swelling in her right breast, and in October, she was diagnosed with BIA-ALCL, following which she removed the implants surgically a month later. The lawsuit claims that the couple and her surgeon were never warned of the risk associated with the textured breast implants.
The lawsuit further states that the implants were made by Silimed, and the manufacturer had acknowledged its failure to follow good manufacturing practices in a first-quarter report published in 2015. These practices result in negligently manufactured, overly textured, rough implant shells containing foreign and adulterated silicone particles, fragments, implant materials, and residues, which triggers T-cell lymphoma and over time, BIA-ALCL, the lawsuit claims.
The couple is seeking damages based on negligence, manufacturing defects, failure to warn, and breach of express and implied warranties for the pain and suffering, and the permanent physical impairment and disfigurement caused, along with punitive damages.
Allergan is also facing similar lawsuits over its recalled “Biocell” textured breast implants. The Food and Drug Administration (FDA) recently indicated that out of the 733 BIA-ALCL cases reported, where the manufacturer has been identified, 620 are linked to breast implants sold by Allergan.
J&J's First $100M Settlement Over Talcum Powder Lawsuits

Johnson & Johnson (J&J), the pharmaceutical giant, has agreed to pay over $100 million to resolve about 1,000 lawsuits that allege its Baby Powder and other talcum powder products for causing cancer.
The settlement news, published by Bloomberg News, states that the deal will resolve claims brought by at least three law firms, which include Simmons Hanly Conroy, Simon Greenstone Panatier PC, and the Lanier Law Firm.
This is the first major settlement announced by J&J since the MDL was formed four years back, which now comprises of nearly 20,000 Baby Powder lawsuits and Shower-to-Shower lawsuits. The lawsuits are mostly filed by women who claim that they were exposed to talc and asbestos particles present in J&J's popular products, which resulted in ovarian cancer and other injuries.
Kim Montagnino, a J&J spokeswoman, maintained in an email that the company's talc products are safe and are backed with “scientific evidence” to support its position. She further stated that the company chose to settle the lawsuits but did not admit liability and will not change its take on the safety of the products.
Last month, U.S. District Judge Freda Wolfson, overseeing the talcum powder litigation, rejected J&J's request to appoint court-approved experts to assist jurors in upcoming bellwether trials, stating that the move would interfere with the independent assessment of the evidence by the jurors. The rejection cleared the way for individual bellwether trials to go before the juries in the federal court system.
In July, Bloomberg Intelligence suggested that to resolve the entire litigation J&J would have to pay as much as $10 billion.
Federal Judge Clears Boehringer Over Pradaxa Death Lawsuit
Last week, Judge William Ray II of the U.S. District Court for the Northern District of Georgia issued an order concluding that Boehringer Ingelheim Pharmaceuticals Inc. has been awarded summary judgment over a Pradaxa wrongful death lawsuit that claimed the manufacturer failed to warn about the bleeding risks associated with the blood thinner.
The lawsuit, filed by Kimberly A. Lyons, on behalf of the estate of Cora Underwood, states that in 2016, Underwood, 75, was prescribed Pradaxa as per the recommended dosing instructions. Underwood was admitted to the hospital after suffering from Cardiac tamponade, which resulted in her death. Cardiac tamponade is a serious medical condition in which blood or fluids around the heart fills up, interfering with its functioning.
Lyons, in the suit, asserted that the cause of death was the high concentration of Pradaxa in the blood, which was the result of age and use of P-gb inhibitors. Boehringer was accused of failing to warn about the increased risk associated with age and other medications and for not recommending plasma monitoring to avoid such events. A paper published and sent to the Food and Drug Administration (FDA) in 2014 by Boehringer scientist Paul Reilly was also pointed to support the plaintiff's claims, but Judge Ray noted that the FDA was aware of the link between concentration levels and bleeding risks, which was mentioned in the paper.
Lyons also argued over the difference in European label for Pradaxa and pushed a design defect claim, both of which were rejected by the judge.
A spokesperson for Boehringer Ingelheim stated that plaintiffs' claims are preempted by six separate decisions from four different federal and state court judges based on the company's study on Pradaxa and data-sharing with the FDA, and the company considers that this decision should be applied on all other Pradaxa cases in its favor.
Couple Wins Over $2.5M In Calif.'s Virtual Asbestos Trial
Last week, the California Superior Court for Alameda County jury awarded a retired Navy admiral and his wife over $2.5 million in the conclusion of a virtual asbestos trial against Metalclad Insulation LLC.
The September 28th verdict was the result of hearing arguments for two-and-a-half months and more than a week of deliberations. A 12-member jury held the lone remaining defendant Metalclad just seven percent liable for negligently failing to recall or warn about the risks associated with the asbestos-laden insulation, which the company supplied to Navy yards for years.
The lawsuit was filed by 82-year-old retired Rear Adm. Ronald Wilgenbusch, and his wife, Judith, claiming that the asbestos exposure during the installation and removal of Metalclad-supplied insulation on several Navy ships in the U.S. Navy caused his mesothelioma. Wilgenbusch will roughly receive $1.8 million, and his wife will receive $750,000 in economic and non-economic damages.
The jury cleared Metalclad of the punitive damages claim and held the Navy 51% liable. Wilgenbusch also bored 4% of the liability, and the other ten companies were held accountable for the rest.
The case, originally headed for an in-person trial in July, was moved for an online trial via Zoom by Alameda County Superior Court Judge Brad Seligman, after finding that one juror came down with a fever.
It is the second of the three that went for trial via Zoom in Alameda County. The first one ended in a defense verdict for Honeywell over a $70 million asbestos suit. The third one kicked off a couple of weeks ago with an argument by a former Navy sailor's counsel that the plaintiff was exposed to cancer-causing asbestos, installed on a Navy aircraft carrier, which was supplied by Metalclad.

