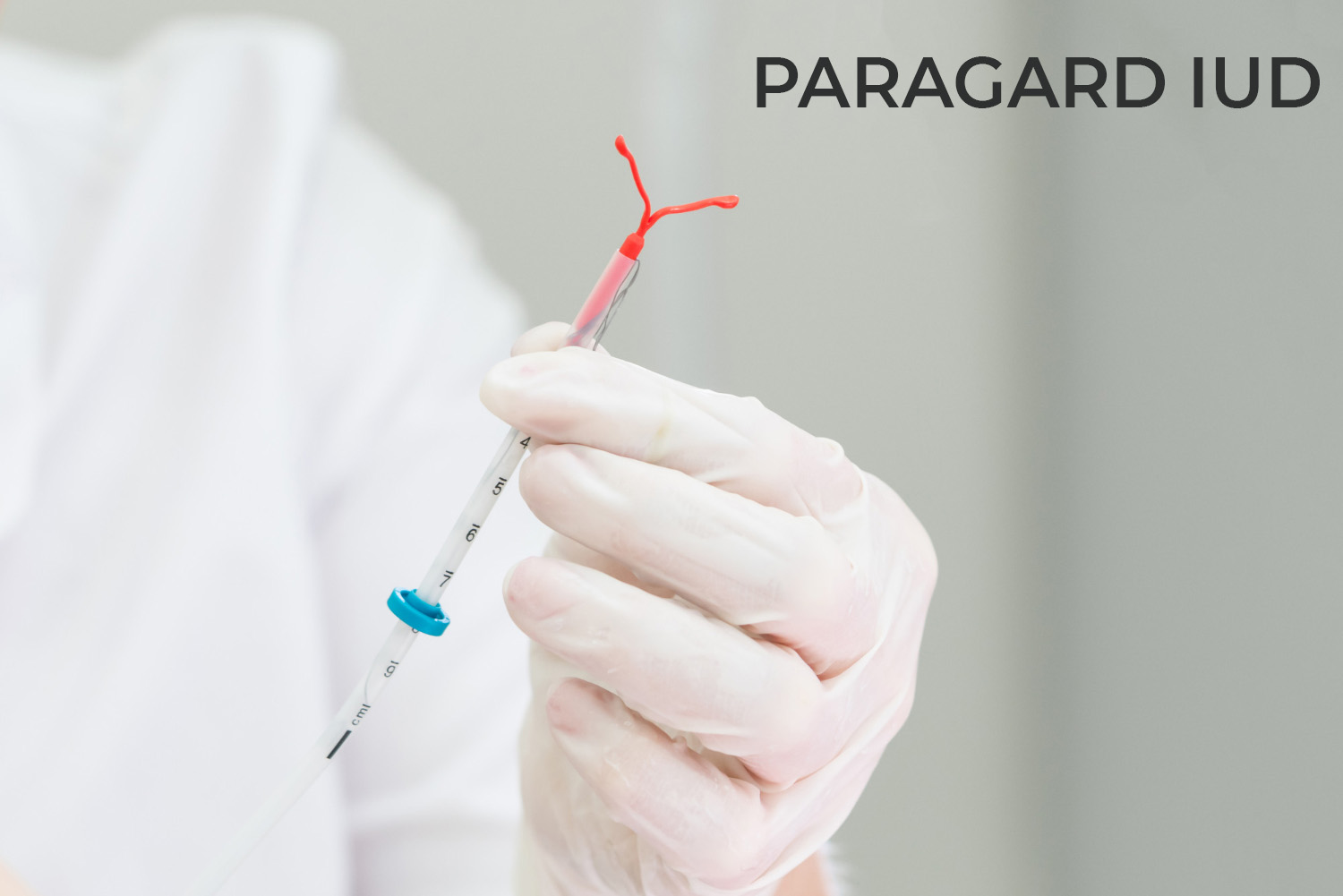
ParaGard, a small IUD (intrauterine device) that is used to prevent pregnancy works differently using one simple active ingredient, copper, instead of hormones, unlike its other counterparts. It is a T-shaped device and can help avoid pregnancy for up to 10 years.
The copper present in the device is continuously released into the uterine cavity, which interferes with sperm transport or fertilization, and the prevention of implantation.
The use of IUD results in less than 1 pregnancy per 100 women each year as per the pregnancy rate in clinical studies. The FDA approved five IUD brands for sale in the U.S.: Mirena, Kyleena, Liletta, Skyla, and ParaGuard.
IUD manufactured by Finishing Enterprises Inc. was FDA-approved on 11/15/1984. The device was approved for use of only 4 years, but the use was extended to 10 years in 1994.
Common Side Effects:
- Anemia
- Backache
- Dysmenorrhea
- Dyspareunia
- Expulsion, complete or partial
- Leukorrhea
- Menstrual flow, prolonged
- Menstrual spotting
- Pain and cramping
- Urticarial allergic skin reaction
- Vaginitis
Studies That Strengthen The Link Of Associated Risks:
October 2020: A study was published in the Journal of Health Sciences and Medicine, in which Turkish researchers reported a link between pelvic inflammatory disease and copper IUDs, like ParaGard.
A cross-sectional study was conducted by the researchers on examination findings, microbiological and pathological evaluations, and other data from a hospital database of 974 patients. The researchers revealed that the use of a Copper-intrauterine Device (CU-IUD) and Levonorgesterone IUD (Mirena) affects the bacterial environment, which results in the development or prevention of pelvic inflammatory disease.
Serious Alleged Injuries May Include:
- IUD “Stuck” In The Uterus
- Perforation Of The Uterus
- Migration Of The Device
- Device Breakage Leading To Surgery
- Copper Wire Left Behind In The Body, Potentially Causing Inflammation And Injury
- Infections
- Scarring
- Organ Damage
- Ectopic Pregnancy
FDA Safety Warnings:
April 2014: A batch of ParaGard T 380A Intrauterine Copper Contraceptive devices were recalled due to a lack of assurance of sterility by the FDA.
The FDA has given the following Warnings & Instruction over the use of ParaGard:
Ectopic Pregnancy: Promptly evaluate women who become pregnant for ectopic pregnancy while using ParaGard.
Risks with Intrauterine Pregnancy: Increased risk of spontaneous abortion, septic abortion, premature delivery, sepsis, septic shock, and death if pregnancy occurs. Remove ParaGard if pregnancy occurs with the device in place.
Sepsis: Group A streptococcal infection has been reported; the strict aseptic technique is essential during insertion.
Pelvic Inflammatory Disease (PID) and Endometritis: Before using ParaGard, consider the risks of PID and endometritis. Promptly assess and treat patients with signs and symptoms of PID.
Embedment: Surgical removal may be necessary.
Perforation: May reduce contraceptive effectiveness and require surgery. Risk is increased if inserted in lactating women and may be increased if inserted in women with fixed, retroverted uteri or convoluted uteri.
Expulsion: Partial or complete expulsion may occur. Remove a partially expelled ParaGard.
Bleeding patterns: Maybe altered and result in heavier and longer bleeding with spotting.
MRI Safety Information: Patients using ParaGard can be safely scanned with MRI only under certain conditions.
Legal Updates:
Defendants:
Teva Pharmaceuticals
Defendant Law Firm:
Teva is represented by Ulmer & Berne LLP.
Allegations: Lawsuits allege that the manufacturer failed to provide adequate warning about the risks of the ParaGard breaking and potentially perforating the uterus.
Lawsuit Status: Several lawsuits have been filed against Teva charging with failure to warn, negligence, breach of warranty, misrepresentation, and designing a defective product. The U.S. Judicial Panel on Multidistrict Litigation (JPML) issued a transfer order in December 2020, confirming centralization in the Northern District of Georgia.
MDL No. 2974 (IN RE: ParaGard IUD Products Liability Litigation)
Presiding Judge: Honorable Judge Leigh Martin May of the United States District Court for the Northern District of Georgia.
MDL Formation Date: 12/16/2020
MDL Status: Active and on appeal.
Important Legal Proceedings:
December 2020: The U.S. Judicial Panel on Multidistrict Litigation (JPML) issued a transfer order, confirming centralization in the Northern District of Georgia.
November 2020: Teva Pharmaceuticals filed a notice over the removal of a single ParaGard IUD lawsuit to the Eastern District of Pennsylvania, which was originally filed in the Philadelphia County Court of Common Pleas.
October 2020: Teva Pharmaceuticals and The Cooper Companies, Inc. filed a motion asking the JPML to reject the consolidation of all ParaGard IUD lawsuits.
September 2020: The plaintiffs involved in the ParaGard IUD lawsuits filed a motion asking the JPML to consolidate the cases before one judge in the U.S. District Court for the Central District of California.
News:
Feb 10, 2021: - NE Woman's ParaGard Warning Claims Refused To Be Revived:
On Monday, the 8th Circuit U.S. Court of Appeals affirmed that Teva Pharmaceuticals, the manufacturer of ParaGard intrauterine contraceptive device (IUD), was not required to warn a woman directly over the risks associated with using the birth control device as the company warned her physician.
The lawsuit was filed by a Nebraska woman after her physicians discovered that the device had broken apart and a piece had become embedded in her uterus. The plaintiff in her lawsuit had cited a Massachusetts case and two cases from Michigan in which the consumers had to be directly warned about the risks for prescription contraceptives.
According to the opinion dated February 8, the decision was a split where the panel majority asserted that like any other state, Nebraska requires manufacturers to warn consumers directly about any design risks in their products. However, there is an exception called the learned-intermediary doctrine for prescription drugs, as per which the manufacturers are allowed to warn medical professionals of the risks, instead of the patients themselves.
The Nebraska Supreme Court did not state whether it would apply the doctrine to other medical products like ParaGard, but the panel said that the court would do it.
U.S. Circuit Judge Jane Kelly, in a dissent, stated that the panel should not venture into an uncertain area of tort law without seeking guidance from the Nebraska Supreme Court. She further added that the question of whether the learned-intermediary doctrine covers prescription contraceptives should be sent to the Nebraska Supreme Court.
In December, the U.S. Judicial Panel on Multidistrict Litigation (JPML) issued a transfer order confirming the centralization of all ParaGard IUD cases in the Northern District of Georgia. The cases will be presided by Honorable Leigh Martin May for coordinated or consolidated pretrial proceedings.
Feb 27, 2021 - FDA Issues Warning Letter Over A ParaGard IUD Advertisement:
On Tuesday, the United States Food and Drug Administration (FDA) issued a warning letter to CooperSurgical, Inc., stating that the manufacturer did not communicate risk information associated with the ParaGard IUD birth control device in a direct-to-consumer video advertisement.
The warning letter states that the advertisement was titled “ParaGard: Family Planning During the Pandemic” and aired on WBTS's The Hub Today on October 5, 2020.
According to the letter, the advertisement contained false or misleading information, and the manufacturer did not submit it to the FDA for review as per the Office of Prescription Drug Promotion (OPDP). The letter warns that this misleading presentation is particularly concerning from a public health perspective due to the serious and potentially life-threatening risks associated with the device, such as those contained in the WARNINGS AND PRECAUTIONS section of ParaGard's PI (prescriber information). The manufacturer is also asked to submit a comprehensive plan to disseminate truthful, non-misleading, and complete corrective communications about the concerns mentioned in the warning letter.
Meanwhile, Judge Leigh Martin May of the U.S. District Court for the Northern District of Georgia, presiding over the multidistrict litigation (MDL) docket for ParaGard IUD injury cases, appointed a group of 25 plaintiffs lawyers for various leadership roles.
The docket was formed in December 2020 when the U.S. Judicial Panel on Multidistrict Litigation (JPML) issued a transfer order confirming the centralization of all ParaGard IUD cases in the Northern District of Georgia for coordinated or consolidated pretrial proceedings.
Currently, around 116 complaints are pending in the MDL and the number of lawsuits seems to be growing, each claiming that the birth control device has a risk of breaking upon removal, causing complications and injuries, including surgeries to remove the broken piece of the device, infertility, and pain.
Mar 22, 2021 - ParaGard MDL: Master Complaint Proposed For Direct Filing:
On March 09, Judge Leigh Martin May of the U.S. District Court for the Northern District of Georgia, presiding over all ParaGard IUD injury cases, issued a case management order, asking the plaintiff to file a “Master Complaint” this month, which will allow the direct filing of future claims through a “Short Form Complaint.”
According to the order, the parties are required to meet and prepare an agreed-upon Short Form Complaint, along with a Direct File Order to streamline the process of filing new claims directly in the Northern District of Georgia.
The plaintiffs are required to file the Master Complaint by today and the defendants are also required to file any responsive pleadings by May 6, 2021.
The process is intended to assist the parties coordinate, categorize and evaluate the claims and will also help avoid costs and delays associated with transferring claims from U.S. District Courts nationwide.
Currently, around 116 complaints are pending in the multidistrict litigation (MDL) and the number of lawsuits seems to be growing, each claiming that the birth control device has a risk of breaking upon removal, causing complications and injuries, including surgeries to remove the broken piece of the device, infertility, and pain.
The MDL docket was formed in December 2020 when the U.S. Judicial Panel on Multidistrict Litigation (JPML) issued a transfer order confirming the centralization of all ParaGard IUD cases in the Northern District of Georgia for coordinated or consolidated pretrial proceedings.
Last month, the United States Food and Drug Administration (FDA) issued a warning letter to CooperSurgical, Inc., stating that the manufacturer did not communicate risk information associated with the ParaGard IUD birth control device in a direct-to-consumer video advertisement.
According to the letter, the advertisement contained false or misleading information, and the manufacturer did not submit it to the FDA for review as per the Office of Prescription Drug Promotion (OPDP). The letter warns that this misleading presentation is particularly concerning from a public health perspective due to the serious and potentially life-threatening risks associated with the device, such as those contained in the WARNINGS AND PRECAUTIONS section of ParaGard's PI (prescriber information).
Evidence:
- Duration Of Usage
- Indication Of Usage In Medical Records
- Evidence Of Injury In Follow Up Medical Records
Medical Record Review and claim validation of ParaGard IUD case should take approximately 3 hours in most instances; however, this approximation may vary in cases based on the volume of records.